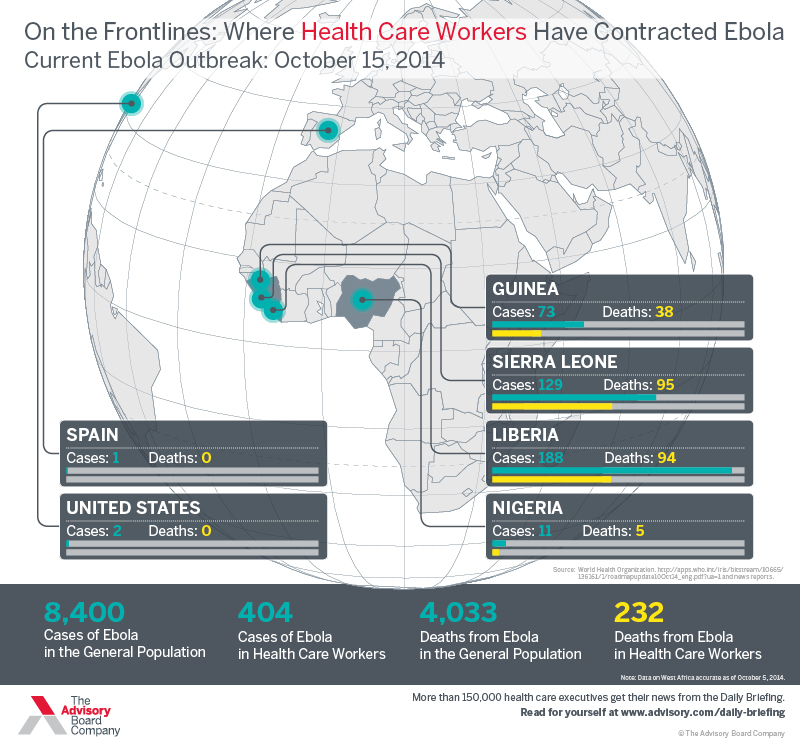
Ebola has now infected multiple people in America: The first two homegrown cases of Ebola are a pair of nurses who got sick after treating Thomas Duncan, the first person ever diagnosed with Ebola in the United States.
Both nurses with Ebola — Nina Pham and Amber Joy Vinson — were among the 70-plus staff who encountered Duncan during his 10-day stay at Dallas’s Texas Health Presbyterian Hospital. However, several staff on Wednesday anonymously told reporters that the nurses were not given sufficient protection against the risk of Ebola.
(A person can become infected with Ebola after being in close proximity with a visibly sick Ebola patient, and specifically by making contact with an infected person’s bodily fluids.)
Although Duncan was admitted to the hospital on September 28, having traveled from West Africa and suffering from common Ebola symptoms like vomiting and diarrhea, staff weren’t told to wear protective gear to guard themselves against Ebola until September 30. That’s when tests formally confirmed that Duncan was infected with Ebola, and had been contagious for nearly a week.
The allegations that the Dallas nurses didn’t have sufficient protections against Ebola are disturbing — more on those in a second — but the infection pattern keeps with a sad trend: A disproportionate number of people who were sickened with Ebola in West Africa were health care workers, too.
Read more – Forbes
October 15, 2014 | Greg

While GlaxoSmithKline’s new drugs Anoro and Breo have yet to take flight like the company had hoped, the drugmaker ($GSK) has been expanding the North Carolina plant where it makes them and other respiratory drugs, expecting that they will.
The U.K. company has invested about $90 million in the plant in Zebulon, where those drugs and two other more recently approved respiratory meds, Incruse and Arnuity, are made, along with the Ellipta inhaler that dispenses them, the Triangle Business Journal reports. Those dollars have covered everything from plant expansion to equipment and some raw materials.
Read more – FiercePharma Manufacturing
October 15, 2014 | Greg
The hospital that treated Ebola victim Thomas Eric Duncan had to learn on the fly how to control the deadly virus, adding new layers of protective gear for workers in what became a losing battle to keep the contagion from spreading, a top official with the Centers for Disease Control and Prevention said Tuesday.
“They kept adding more protective equipment as the patient [Duncan] deteriorated. They had masks first, then face shields, then the positive-pressure respirator. They added a second pair of gloves,” said Pierre Rollin, a CDC epidemiologist.
Despite the infection-control efforts, a nurse, Nina Pham, 26, somehow contracted Ebola at Texas Health Presbyterian Hospital Dallas while caring for Duncan, a Liberian man who flew to the United States last month. Pham is being treated at the same hospital, and Tuesday she was reported to be in good condition. (On Wednesday, state officials announced that a second worker who cared for Duncan had also tested positive for the virus.)
CDC Director Thomas Frieden expressed regret Tuesday that his agency had not done more to help the hospital control the infection. He said that, from now on, “Ebola response teams” will travel within hours to any hospital in the United States with a confirmed Ebola case. Already, one of those teams is in Texas and has put in place a site-manager system, requiring that someone monitor the use of personal protective equipment.
Read more – The Washington Post
October 15, 2014 | Greg

A second health worker in the US state of Texas has tested positive for Ebola, health officials say.
Both health workers treated Liberian man Thomas Duncan, who died last week after becoming the first person diagnosed with Ebola in the US.
Meanwhile, the UN’s Ebola mission chief says the world is falling behind in the race to contain the virus.
The World Health Organization (WHO) says 4,447 people have died from the outbreak, mainly in West Africa.
Read more – BBC News
October 15, 2014 | Greg
We are all alarmed by the scope and scale of the human tragedy occurring in West African nations affected by the Ebola virus disease epidemic. While the cornerstones of the Ebola response remain prompt diagnosis and isolation of patients, tracing of contacts, and proper protective equipment for healthcare workers, the National Institutes of Health (NIH), led by its National Institute of Allergy and Infectious Diseases (NIAID), is spearheading efforts to develop treatments and a vaccine for Ebola as quickly as possible.
For example, NIAID has supported and collaborated with Mapp Biopharmaceutical, Inc., San Diego, in its development of the product known as ZMapp, which has been administered experimentally to several Ebola-infected patients. While it is not possible at this time to determine whether ZMapp benefited these patients, NIAID is supporting a broader effort to advance development and clinical testing of ZMapp to determine if it is safe and effective. In addition, the U.S. Biodefense Advanced Research and Development Agency (BARDA) has announced plans to optimize and accelerate the manufacturing of ZMapp, which is in limited supply, to enable clinical safety testing to proceed as soon as possible.
NIAID is also supporting the development of other potential treatments. Among them is BCX4430, a drug that BioCryst Pharmaceuticals, Research Triangle Park, NC, plans to enter into a Phase 1 human clinical trial by early 2015. Another potential drug, brincidofovir, developed by Chimerix of Durham, N.C., has been evaluated in more than 1,000 patients for two non-Ebola viruses (cytomegalovirus, adenovirus). Brincidofovir has shown some ability to suppress Ebola viruses in cell cultures, and was recently administered to several patients with Ebola virus disease. Again, while it is impossible to determine on the basis of a few patients whether a drug is effective against Ebola, brincidofovir appears to be safe in humans. Broader evaluation of the drug will likely take place in the coming months.
Read more – NIH Director’s Blog
October 14, 2014 | Greg
Within days of Prime Minister Narendra Modi’s return from the US, the US Trade Representative (USTR) has launched a fresh offensive against India’s intellectual property rights (IPR) regime, a move that may lead to the government going slow on a bilateral dialogue with Washington.
On Tuesday, USTR will launch what it calls an “out-of-cycle review” (OCR) of India’s IPR regime, following a report released earlier this year, where India was put on the priority watch list due to “heightened level of concern about the deterioration in India’s environment for IP protection and enforcement”. Now, it is seeking public comments and information related to law, policies, or practices related to India’s IPR.
The US government has been under pressure from local lobby groups, led by pharma multinationals, to initiate action as the Indian law has special provisions that allow patent authorities to reject a patent application citing lack of innovation. Big Pharma is particularly upset with the use of compulsory licensing provisions that allowed authorities in India to waive the patent rights for a cancer drug and fear that other countries may follow suit to make drugs more affordable for patients.
Read more – The Times of India
October 13, 2014 | Greg

The US Congress move to investigate the price rise in certain generic drugs by 14 companies, including those from India, has important lessons for the Indian pharmaceutical industry. Indian drug companies need to realise that they operate in price-sensitive markets – be it India or the US where the demographic of the Baby Boomer generation is witnessing record numbers signing up for MediCare and the Affordable Care Act (ObamaCare).
Therefore, better earnings will only have to come from focus on building internal efficiencies and process innovations. The investigation, though still at a preliminary stage of seeking responses and details from companies, cannot arguably be seen as an anti-India lobby at work, since only three of the 14 companies that have been issued letters (seeking reasons for the price hike in the last one year) are Indian.
Since majority of the firms are headquartered in the US and only a couple of them elsewhere, there is little likelihood of this being seen as a move directed against Indian firms. In certain cases (Doxycycline Hyclate, for example), the products cited were in deep shortage conditions and the market responded with relatively dramatic price increases. This would raise eyebrows in any country, more so in the United States today, where the Affordable Care Act is under siege.
Read more – Business Today
October 9, 2014 | Greg

India’s pharma majors Dr Reddy’s and Sun Pharma are among 14 drug makers that are being probed by the US Congress over price escalation of generic drugs.
In letters sent by Elijah E Cummings (Ranking Member of the House Committee on Oversight and Government Reform), and senator Bernard Sanders, 14 companies have been asked to share “information about the escalating prices they have been charging for generic drugs”.
Cummings and Sanders are investigating price increases for drugs used to treat everything from common medical conditions to life-threatening illnesses and to identify measures to help reduce costs for patients, healthcare providers, and hospitals across the country (US), said a statement on the US House of Representatives website.
In their letters, Cummings and Sanders cited data from the Healthcare Supply Chain Association on recent purchases of 10 generic drugs by group purchasing organisations over the past two years.
“When you see how much the prices of these drugs have increased just over the past year, it’s staggering, and we want to know why,” said Cummings. “In some cases these outrageous price hikes are preventing patients from getting the drugs they need,” he added.
Read more – The Times of India
October 9, 2014 | Greg

After a meeting last week between President Barack Obama and the recently elected Indian Prime Minister Narenrda Modi, the White House issued a statement that included a brief passage saying a new “high level” working group on intellectual property was being created.
The statement prompted concern from advocacy groups that closely track the contentious interplay between access to affordable medicines and pharmaceutical patents. In their view, the initiative may become a tool for pressuring India to change its approach to patent law
Why? India is something of a pharmacy to the world, because so many generic drug makers are based there. Moreover, India has also irked brand-name drug makers with laws and court rulings that have made it easier for their generic rivals to sell lower-cost, copycat versions of their medicines.
This has generated concern in Washington…
Read more – Pharmalot – WSJ
October 8, 2014 | Greg
India’s new Prime Minister, Narendra Modi and his delegation, who have been visiting the U.S. for the first time, have spent considerable time and energy in courting U.S. business interests. On Monday, September 29, the Indian PM met with 17 chief executives of major U.S. companies in joint and individual meetings and on September 30 he met with the U.S.-India Business Council comprising over 300 top U.S. companies. Prime Minister Modi is promising to open India to more direct foreign investment and to further liberalize the Indian economy to make it easier for multinational corporations to operate there. To the dismay of health activists worldwide, the US administration appears to have successfully used the Indian PM’s visit to maneuver the Indian government into committing to a joint mechanism on intellectual property. The benign sounding “High Level Intellectual Property (IP) Working Group” is designed to pressure India into changing its interpretation and application of health safeguards in India’s intellectual property policy, ultimately undermining India’s role as the pharmacy for the poor.
Even before the PM’s visit, alarm bells have been ringing within the global health community over statements made by India’s Minister of Commerce and Industry, Nirmala Sitharaman. The relentless public and private pressure from both the U.S. government and Big Pharma, appear to have prompted the Commerce Minister to announce a review of India’s IP policy with the aim of boosting innovation, improving administrative procedures, and potentially strengthening the country’s patent regime. In announcing the creation of a think tank to make the patent system “more robust,” Minister Sitharaman specifically mentioned that “developed nations are picking holes in India’s IPR laws.”
Read more – infojustice
October 6, 2014 | Greg

India’s biotechnology industry is on the cusp of entering a new era as it already has many of the necessities required to grow its bio economy such as a talented and enthusiastic scientific workforce, a top biotech industry leadership has said.
“In 2012, Indian industry and government leaders set an ambitious yet attainable goal of growing the nation’s bio economy to more than $100 billion by 2025,” Jim Greenwood, CEO Biotechnology Industry Organization (BIO) said yesterday. “BIO believes that the Indian biotechnology industry is on the cusp of entering a new era when it can provide significant economic growth and development to the people of India and around the world,” he said.
Read more – dnaindia.com
October 3, 2014 | Greg
Indian CRO GVK Biosciences is working to get back in the good graces of European regulators after inspectors found evidence that its employees doctored clinical trial results.
As India’s Financial Express reports, in May investigators from France’s Agency for Medicines and Health Products Safety examined 9 trials conducted at GVK’s Hyderabad clinical outpost and discovered that the CRO’s workers repeatedly switched out patient ECG scores with those of healthy volunteers.
Now Europe’s Coordination Group for Mutual Recognition and Decentralized Procedures-Human is working to identify which drugs, approved or pending, might have been affected by the issue, according to the FE.
Read more – FierceCRO
October 2, 2014 | Greg
Germany’s Evotec has joined a trio of research projects focused on multiple sclerosis, contributing its developmental know-how to some early-stage R&D.
The hybrid research organization is on tap to help out with a German government-sponsored effort to look at three pathways of treating the inflammatory disease: cytokine regulation, neuroprotection and tolerance induction. Evotec will lend out its drug discovery platform, project-management capabilities and industry connections to each effort, hoping to identify new candidates that go beyond treating the effects of MS and get to the root of the disease.
The projects range in length from 18 months to three years, Evotec said, and carry a total budget of roughly $6.3 million.
For Evotec, which splits its business into the CRO-like EVT Execute and R&D-focused EVT Innovate, the three deals complement its core competency, Chief Scientific Officer Cord Dohrmann said.
Read more – FierceCRO
October 2, 2014 | Greg
Over the past year India has begun to face up to the reality that its regulatory machinery is insufficient to enforce quality standards, leading authorities to commit to hiring more inspectors. Another piece of the plan has been unveiled, with the Central Drug Standard Control Organization CDSCO revealing Indian inspectors are tagging along on inspections run by their international peers.
CDSCO plans to double its headcount and add laboratory capacity by the end of 2017, but there is little benefit to having 1,000 inspectors unless they know how to assess if a plant is following good manufacturing practices. In the last financial year CDSCO ran 17 training programs for inspectors, Pharmabiz reports, and has worked with the FDA on four GMP workshops on topics including process validation and enforcements. To support these efforts, CDSCO is sending its inspectors into the field with their global peers.
Read more – FiercePharma Manufacturing
October 2, 2014 | Greg

India could run out of a critical medicine in its free HIV/AIDS drugs programme in three weeks due to bureaucratic bungling, a senior government official said, leaving more than 150,000 sufferers without life-saving drugs for about a month.
Missed dosages for long durations can increase patients’ drug resistance and result in faster spread of the virus, while changes in medication regimens expose patients to side effects.
The supply crunch will be an embarrassment for the four-month-old government of Prime Minister Narendra Modi, who has promised to deliver more affordable and better health services.
As drugs in the open market are expensive, the government provides more than one-third of India’s 2.1 million HIV/AIDS patients with free antiretroviral drugs that are procured from pharmaceutical companies via a tender process.
Read more – The Times of India
October 1, 2014 | Greg









