
WHAT could be more welcome in a season that demands fresh starts, abstinence and “detoxing” than to discover that you do not have to lift a finger to avoid cancer? A paper published last week in Science seemed to offer seasonal bingers every reason for ripping up their New Year’s resolutions. According to many reports of the research, it suggested two-thirds of human cancers are caused by nothing more than bad luck.
Read more – The Economist
January 8, 2015 | Greg

While FDA regulatory action against Indian companies has drawn the media spotlight the last couple of years, European regulators also have been finding problems with some Indian plants. Both France and Italy took action against Indian drug manufacturers last month, finding them out of compliance and limiting product sales as a result.
Read more – FiercePharma Manufacturing
January 8, 2015 | Greg

China’s drug regulator has confirmed what the FDA has known for some time. Oversight of pharmaceutical manufacturing in the country that is the major supplier of cheap active pharmaceutical ingredients (APIs) is “weak.” But the China Food and Drug Administration, as it has other times, promised that it will get better in the new year.
Read more – FiercePharma Manufacturing
January 8, 2015 | Greg

A collaborative research team from universities, research institutes, and industry in Boston and Bonn, Germany, reported yesterday on their discovery of a new antibiotic that holds promise – cautious and restrained promise – for not only overcoming antibiotic drug resistance but being, itself, resistance-proof. The drug has yet to be tested in people but shows encouraging results in animals infected with bacteria that cause human disease.
Read more – Forbes
January 8, 2015 | Greg

Before the holidays, the BBC reported on an analysis presented by UK antimicrobial resistance czar, economist Jim O’Neill, saying that drug resistant infections will kill an extra 10 million people a year worldwide – more than currently die from cancer – by 2050 unless action is taken. These infections, commonly called “superbugs” are responsible for 50,000 deaths each year in Europe and the U.S. and that, if unchecked, would spiral to ten times that number by 2050. O’Neill said that scientists seemed more certain that drug resistance would be a bigger problem in the short term than climate change.
Read more – Forbes
January 8, 2015 | Greg
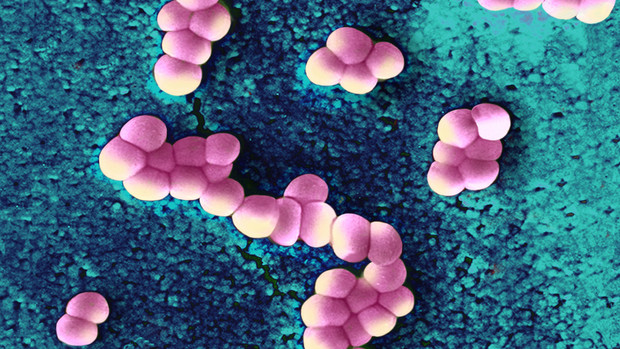
Scientists have discovered an antibiotic capable of fighting infections that kill hundreds of thousands of people each year, a breakthrough that could lead to the field’s first major new drug in more than a quarter-century.
The experimental drug, which was isolated from a sample of New England dirt, is called teixobactin.
Read more – Bloomberg
January 7, 2015 | Greg

After Ranbaxy, three more domestic (Indian) pharmaceutical companies – Lupin, Aurobindo Pharma and Jubilant Life Sciences – have received final approval from the US drug regulator to sell generic valsartan in the US market.
The drug is used to treat hypertension and to lower blood pressure.
Read more – dnaindia.com
January 7, 2015 | Greg
India needs to increase its spending on Research and Development (R & D) to 2%, or Rs. 1,76,000 crore of its Gross Domestic Product (GDP), if the country wants to promote innovation, said Arun Firodia, chairman of Kinetic Group of Companies, who spoke at a symposium on ‘Science and Technology for Inclusive Development’ on the last day of the Indian Science Congress.
Read more – Hindustan Times
January 7, 2015 | Greg
The (Indian) government is introducing the Drugs and Cosmetics (Amendment) Bill to amend the existing Act to consolidate provisions for clinical trials and regulate medical devices.
A recent notification by the Central Drugs Standard Control Organisation regarding the policy initiatives planned for 2015 includes the introduction of the Bill.
Read more – dnaindia.com
January 6, 2015 | Greg
Jubilant Life Sciences has appointed Gurpartap Singh Sachdeva as CEO of Jubilant Pharma, a wholly-owned subsidiary of the pharmaceutical company.
Read more – The Economic Times
January 6, 2015 | Greg

German contract developer Evotec pocketed €8 million ($10 million) from its ongoing R&D collaborations with Bayer and Johnson & Johnson ($JNJ), capping a big year for the innovative company.
Read more – FierceCRO
December 23, 2014 | Greg

Mumbai-based pharmaceutical major Glenmark Pharmaceuticals is strengthening its position in the world’s largest drug market, launching more generic copies. The company, which owns a strong drug molecule pipeline, has been filing a large number of ANDAs (abbreviated new drug applications) of Indian companies with the US Food and Drug Administration (FDA) since FY13.
Read more – Business Standard News
December 22, 2014 | Greg

Stephen M Sammut, Senior Fellow, Health Care Management, Wharton School, University of Pennsylvania and Visiting Faculty, Indian School of Business in an interaction with Shalini Gupta talks about the state of VC environment in India’s biotechnology sector…
Read more – The Financial Express
December 22, 2014 | Greg

The latest World Health Organisation (WHO) initiative on Substandard/Spurious/Falsely-labelled/ Falsified/ Counterfeit medical products has been making slow but steady progress through meetings in 2012, 2013 and the latest on October 29-31, 2014. A technically strong delegation comprising four seniormost drug regulators from (DCSO/DCGI) office represented India.
Read more – The Financial Express
December 22, 2014 | Greg

An analysis of 582 drugs sold in the US by six leading Indian pharmaceutical companies shows that about 86% of these drugs did not register any change in price during calendar years 2013 and 2014, according to a report by Morgan Stanley.
Read more – The Financial Express
December 22, 2014 | Greg












